Unbelievable Info About How To Draw Solubility Curve
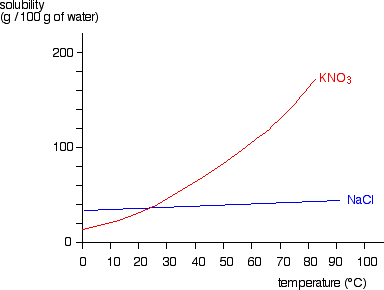
How to draw a solubility curve?explain the below solubility curve?
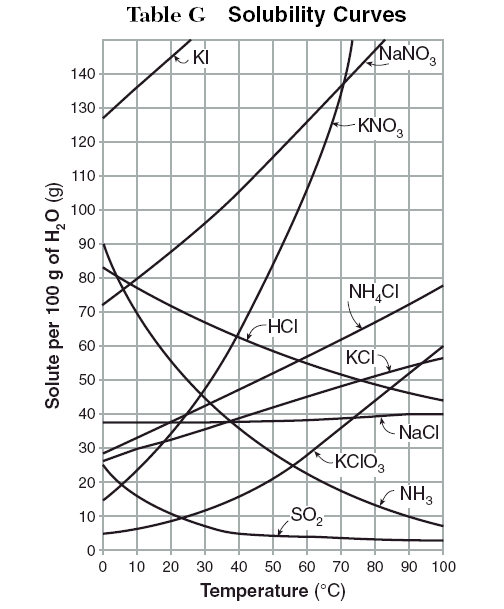
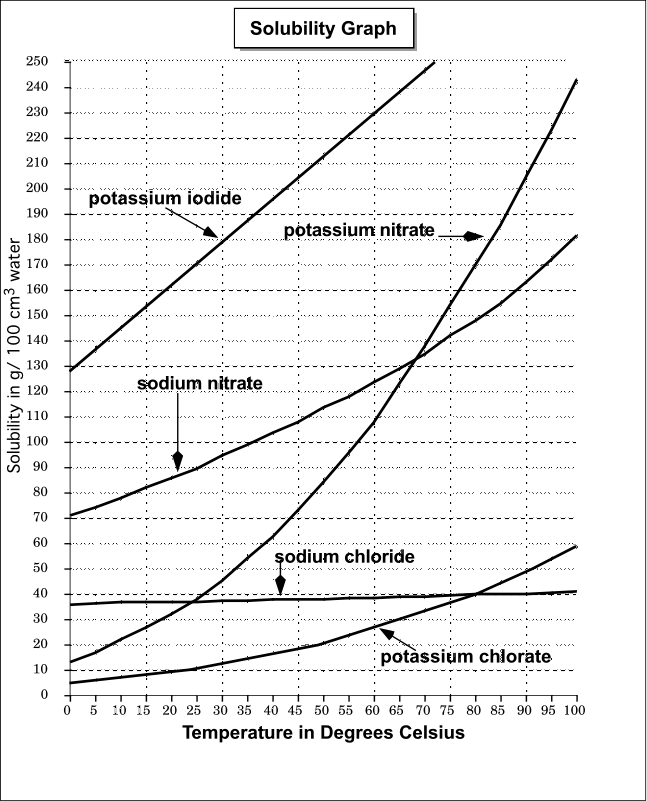
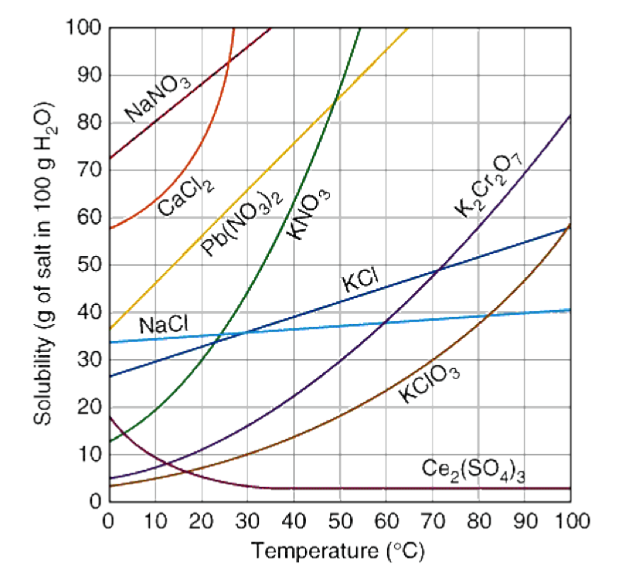
How to draw solubility curve. A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. (i) determine how much solute will dissolve in the solvent at a given temperature. O(ml) solubility(g/100 g) temperature (ºc) 3.
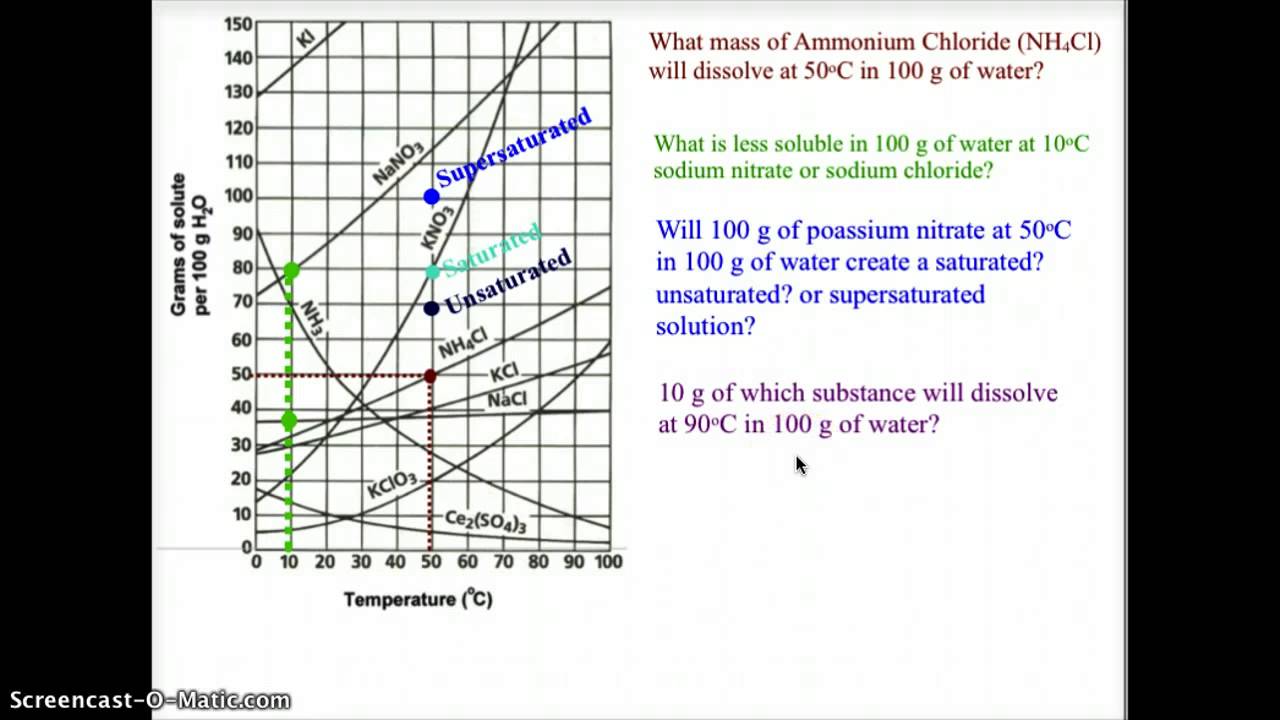
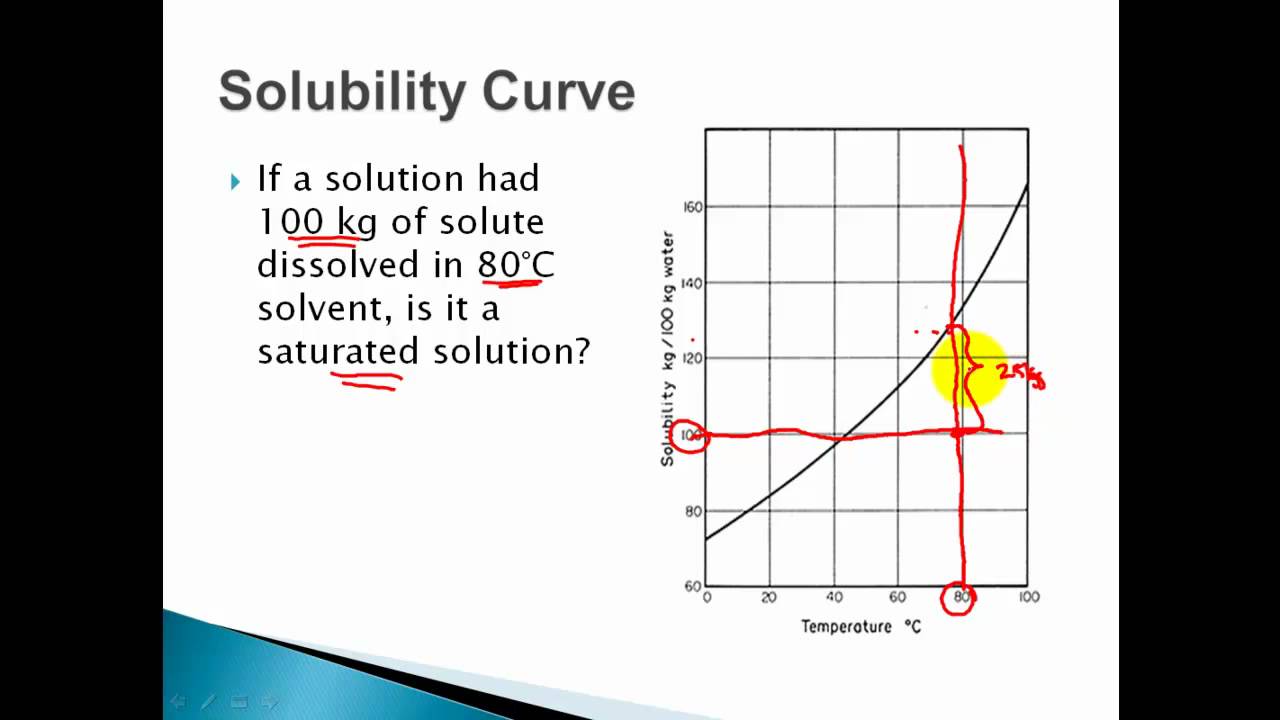
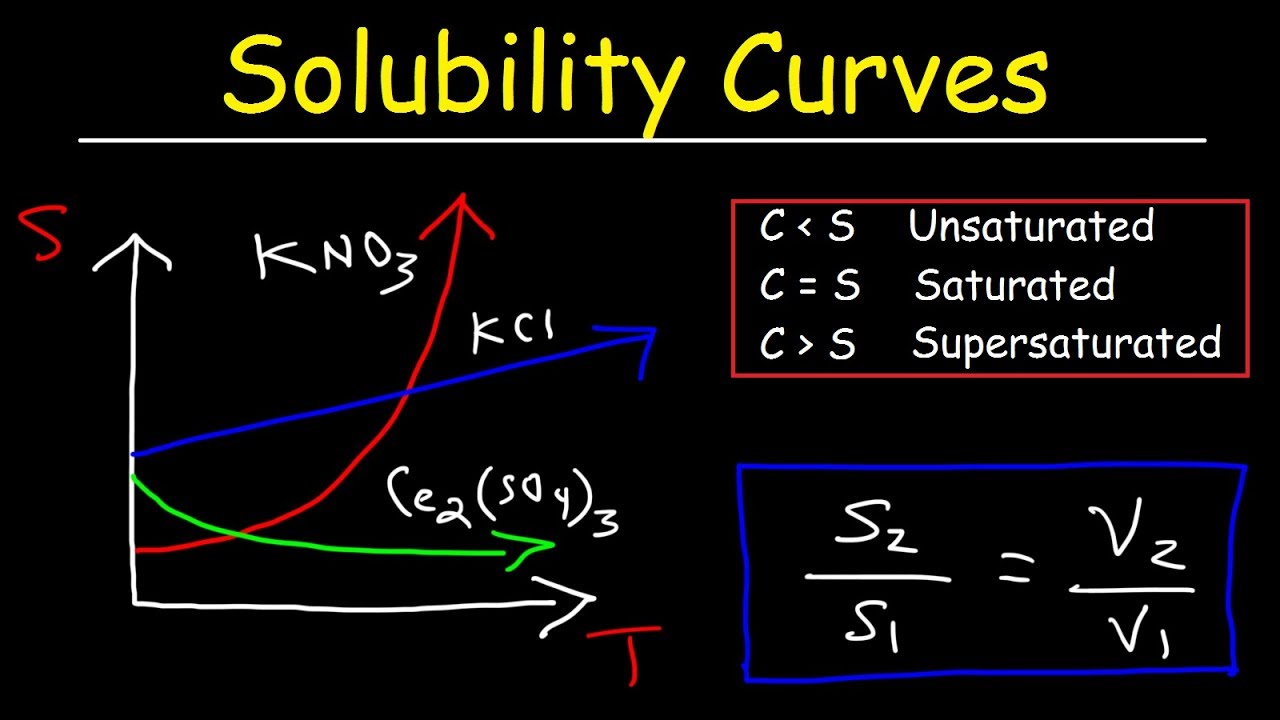
What is a solubility curve? When ready the curve of a particular solute, it is important to see at which temperature the solute creates a saturated solution (on the line), supersaturated solution (above the solute’s solubility. Up to 24% cash back interpreting solubility curve graphs (key) 1.
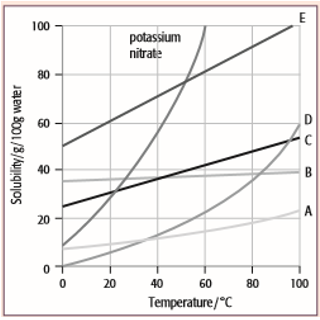
Solubility curves are used to show how the solubility of a substance changes with temperature. Solubility curves can be used to. This video covers how to use solubility curve graphs to determine solubility of substances at specific temperatures, how solubility normally changes with tem.
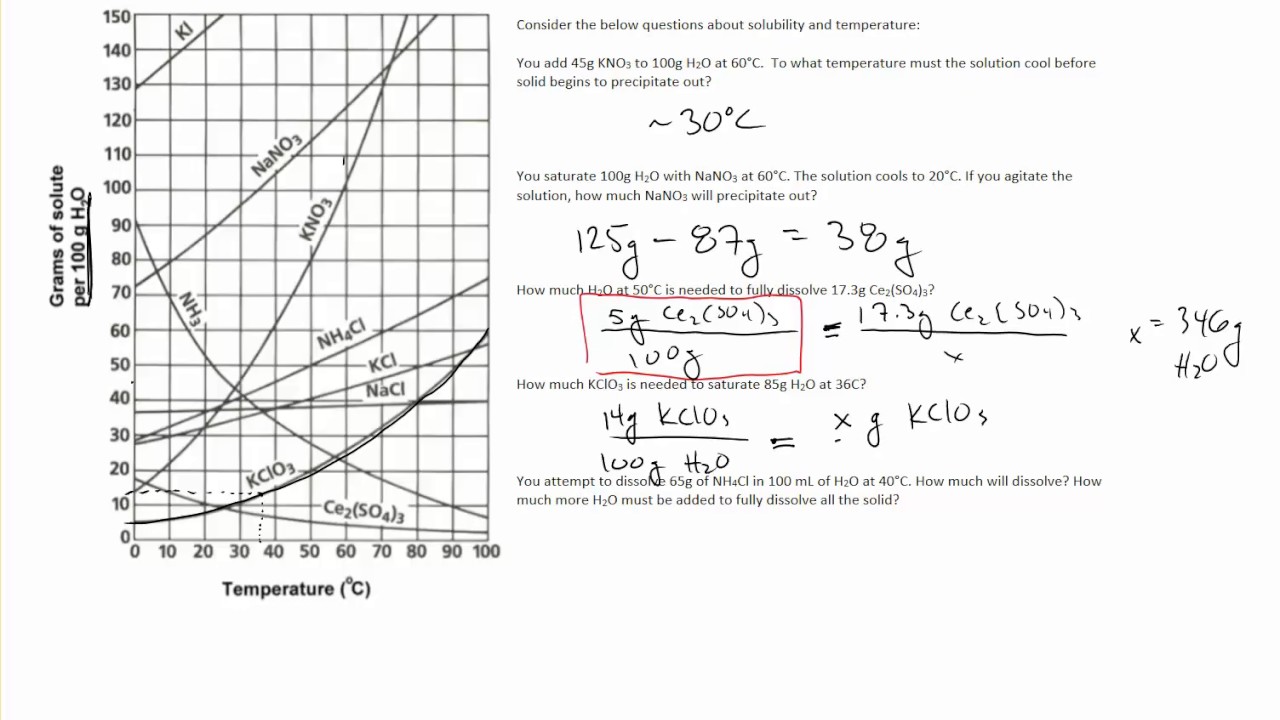
To read the graph, find the line for the substance. One can here see that the solubility. From your solubility curve predict a.
(ii) compare the solubilities of different solutes in the same solvent. Explain nano 3 , because the higher line indicates that more nano 3 can be. At a given ph, the form with the lowest solubility will be the thermodynamically stable one.
Draw a smooth line of best fit to. Which is more soluble nano 3 or kcl? The solubility of ammonium chloride at 600 c.
When ready the curve of a particular solute, it is important to see at which temperature the solute creates a saturated solution (on the line), supersaturated solution (above the solute’s solubility.